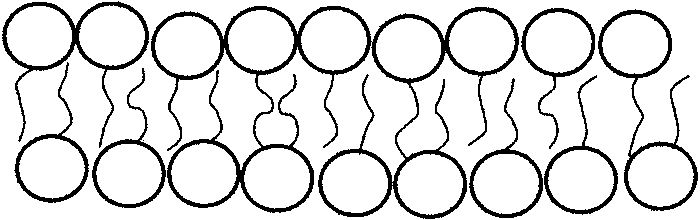
Increasing attention has been devoted to study the behaviour of assemblies of lipid molecules with biological relevance, such as vesicles and tubules (for an update review, see [1]). Each lipid molecule consists of a polar head and a hydrocarbon tail: the former is hydrophilic, whereas the latter is hydrophobic. Thus, in an aqueous environment these molecules tend to be organized in bilayers, each consisting of two molecular layers, with the heads outside and the tails inside. (see Figure 1.1).

Figure 1. Sketch of a bilayer. The hydrophobic tails hide
themselves from the surrounding medium.
Vesicles are usually composed of a single bilayer and can take various equilibrium shapes, all described by closed surfaces: a complete catalogue of vesicles shapes can be found either in [2] or in [3]. Tubules are formed by many bilayers and are open structures, approximately cylindrical. Both vesicles and tubules have a prescribed area, as molecules are not likely to be added to or removed from them. Moreover, in the case of vesicles, the volume enclosed is usually prescribed.
A variational model for the equilibrium of lipid membrane was independently
proposed by Canham in [4] and Helfrich in [5]. They described the
elasticity of a lipid membrane by a quadratic, but not necessarily homogeneous
functional of the curvatures of the membrane. In slightly more general terms, we
can use the following elastic energy for a vesicle: